Pyrosulfuric acid is the main constituent of fuming sulfuric acid and is a strong acid and. It is also known as disulfuric acid or oleum which is an oxyacid of sulfur. Pyrosulfuric acid is weaker than sulfuric acid. Salts such as sodium and potassium pyrosulfates are obtained by reacting with the bases.
The property value of hydrogen bond donor and hydrogen bond acceptor is 2 and 7 respectively. The rotatable bound count is 7. The acid can be synthesised by reacting excess SO3 with sulfuric acid.
Following is the table of formulas of pyrosulfuric acid:
| Molecular formula | H2S2O7 |
| Linear formula | H2O7S2 |
| Simplified molecular-input line-entry system (SMILES) | OS(=O)(=O)OS(=O)(=O)O |
Structure Of Pyrosulfuric Acid
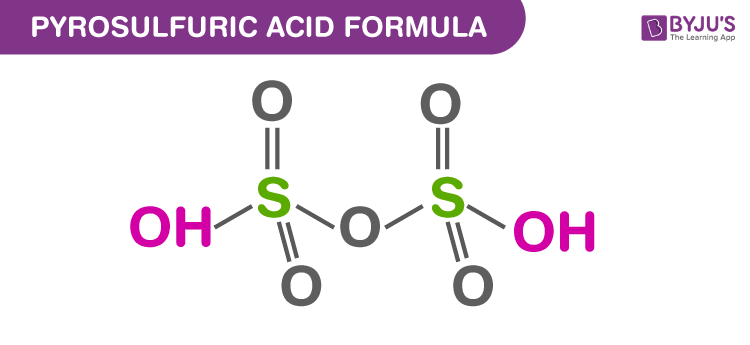
Properties Of Pyrosulfuric Acid
| IUPAC name | sulfo hydrogen sulfate |
| Chemical formula | H2S2O7 |
| Molecular weight | 178.129 g/mol |
| Melting point | 36℃ |
| Conjugate base | Disulfate |
Applications Of Pyrosulfuric Acid
- It is used in the manufacture of an explosive and dyes.
- It is used as a sulfating agent.
- It finds applications in petroleum refining.
The health hazards of pyrosulfuric acid are corrosive to skin and severe eyes irritation when exposed and permanent tissue damage when swallowed.
To learn more about other Chemistry related concepts, stay tuned with BYJU’S.
Comments